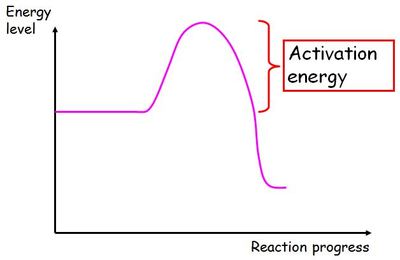
Chemistry Words: Activation energy
Simple Description
The minimum amount of energy needed to start (or "activate") a reaction.
Further Detail
For example, consider striking a match. The match will only light if the match is struck with enough energy - the "activation energy". The amount of energy a reaction needs can be lowered using a catalyst, which helps speed up a reaction.
Related Words:
« Previous Word Next Word »
