Chemistry Words: Groups
Simple Description
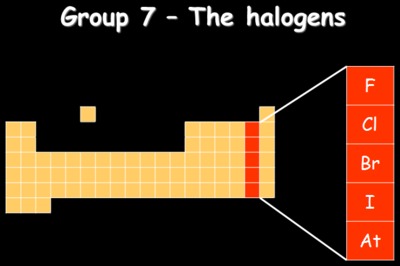
The columns in the Periodic Table, eg group 7 - the halogens
Further Detail
All of the known elements are arranged in groups in the Periodic Table. Each group contains elements that show similar properties; for example, the elements in group 1 (sodium, lithium, potassium etc) are all metal, have low densities and are easy to cut with a knife. The group number also tells you how many electrons are in the outer shell of that element. Group 1 has 1 electron in its outer shell, group 7 have 7 electrons in its outer shell.
Related Words:
« Previous Word Next Word »
