Chemistry Words: Isotope
Simple Description
An atom with the same number of protons but a different number of neutrons
Further Detail
Isotopes have the same number of protons (as this defines the atom) but a different number of neutrons and therefore a different mass. In the example below each isotope has the same number of protons but the mass number is different, indicating that these are isotopes.
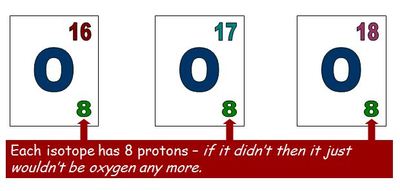
Related Words:
« Previous Word Next Word »
