Chemistry Words: Outer shell
Simple Description
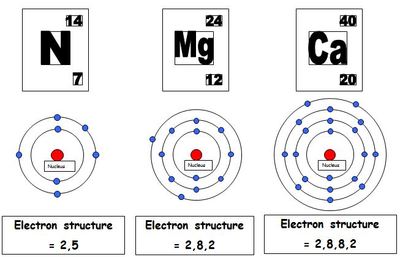
The outermost electron shell in an atom - it usually takes up to 2 or 8 electrons
Further Detail
The number of electrons in the outer shell of an atom matches the group number for that element on the Periodic Table. For example, carbon is in group 4 and has 4 electrons in its outer shell. All atoms want to have a full outer shell (usually 8) so they form bonds in order to do this. The elements in group 8 already have 8 electrons in their outer shell so they do not form bonds - it is very rare to see group 8 elements in a compound.
Related Words:
« Previous Word Next Word »
