Chemistry Words: Percentage yield
Simple Description
The amount (in %) of product made in a reaction compared to what SHOULD have been made
Further Detail
If a reaction has a percentage yield of 100% then everything you predicted to make was actually made. In reality, it is never 100% and this could be because of a number of reasons - maybe some of the reactant or product was lost, maybe the reaction did not carry through to completion etc.
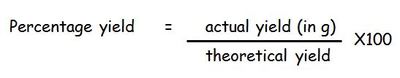
Related Words:
« Previous Word Next Word »
