Chemistry Words: Strong acids
Simple Description
Strong acids are fully ionised in solution
Further Detail
Strong acids such as hydrochloric acid is fully ionised, it has formed H+ ions and Cl- ions. At the same concentration strong acids have a higher concentration of hydrogen ions than weaker acid such as ethanoic acid, which has only partially ionised.
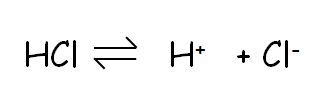
« Previous Word Next Word »
