Physics Words: Metal
Simple Description
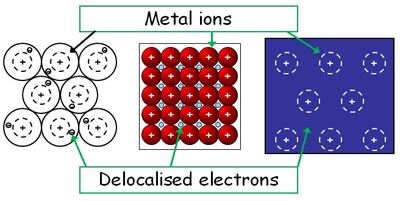
A substance containing delocalised (free) electrons - they are good conductors of heat and electricity
Further Detail
Metals are made of positive ions and delocalised (free) electrons. The positive ions and negative electrons are held together by the attraction between their opposite charges - a "metallic bond". Metals are good conductors of heat and electricity and are usually strong, tough and solid at room temperature (except mercury).
Related Words:
« Previous Word Next Word »
